Embark on a fascinating journey into the realm of df how to make steel, where we unravel the intricate processes and transformative power that forge this remarkable material. From the humble beginnings of iron ore to the cutting-edge advancements in steelmaking, this exploration unveils the secrets behind the creation of a metal that has shaped civilizations and continues to drive progress.
Delving into the depths of steelmaking, we will trace the evolution of techniques, from the pioneering Bessemer process to the sophisticated electric arc furnace method. We will discover the crucial role of alloying elements in tailoring steel’s properties and explore the diverse applications that make steel an indispensable material in countless industries.
1. Raw Materials and Preparation: Df How To Make Steel
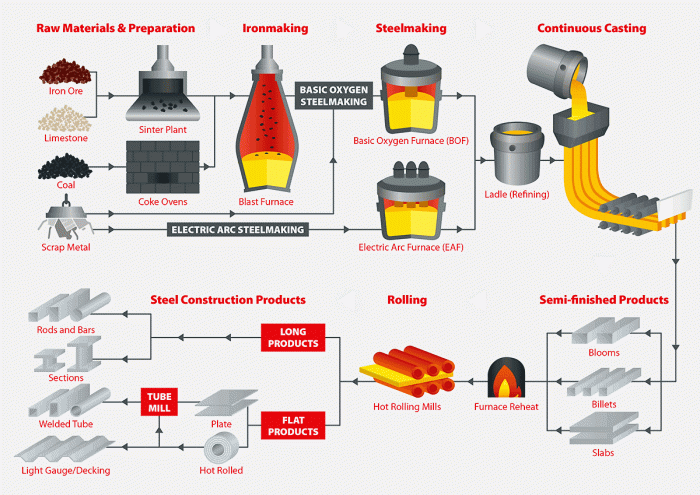
The production of steel begins with the extraction of iron ore, a naturally occurring mineral that contains iron oxides. The most common types of iron ore used for steelmaking are hematite (Fe2O3), magnetite (Fe3O4), and limonite (FeO(OH)·nH2O). These ores are typically mined from open-pit or underground mines.
Once the iron ore has been mined, it is transported to a steel mill, where it is crushed and ground into a fine powder. The powder is then mixed with coke (a form of coal) and limestone (a flux) and heated in a blast furnace.
The coke provides the carbon necessary to reduce the iron oxides to metallic iron, while the limestone removes impurities from the ore.
The molten iron produced in the blast furnace is known as pig iron. Pig iron contains a high level of carbon (about 4%), which makes it brittle and unsuitable for most applications. To convert pig iron into steel, the carbon content must be reduced.
There are several different methods for refining pig iron to produce steel. The most common methods are the Bessemer process, the open-hearth process, and the electric arc furnace process.
2. Steelmaking Processes

The Bessemer process is a relatively old method of steelmaking that was developed in the 1850s. In the Bessemer process, molten pig iron is poured into a large converter, which is lined with a refractory material. Air is then blown into the converter, which oxidizes the carbon and other impurities in the iron.
The oxidized impurities are removed as slag, and the remaining iron is converted into steel.
The open-hearth process is another traditional method of steelmaking that was developed in the late 1800s. In the open-hearth process, molten pig iron is poured into a large furnace, which is lined with refractory bricks. The furnace is heated by burning fuel, and the molten iron is oxidized by air that is drawn into the furnace.
The oxidized impurities are removed as slag, and the remaining iron is converted into steel.
The electric arc furnace process is a more modern method of steelmaking that was developed in the early 1900s. In the electric arc furnace process, molten pig iron is poured into a large furnace, which is lined with refractory bricks.
The furnace is heated by an electric arc, and the molten iron is oxidized by oxygen that is injected into the furnace. The oxidized impurities are removed as slag, and the remaining iron is converted into steel.
Each of these steelmaking processes has its own advantages and disadvantages. The Bessemer process is relatively inexpensive, but it produces a lower quality of steel than the other two processes. The open-hearth process produces a higher quality of steel than the Bessemer process, but it is more expensive.
The electric arc furnace process produces the highest quality of steel, but it is the most expensive of the three processes.
Fluxes are used in steelmaking to remove impurities from the molten iron. The most common fluxes are limestone and dolomite. Limestone is a calcium carbonate (CaCO3), and dolomite is a calcium magnesium carbonate (CaMg(CO3)2). When these fluxes are added to the molten iron, they react with the impurities to form a slag, which floats to the surface of the molten iron and can be easily removed.
3. Alloying and Heat Treatment
Alloying is the process of adding other elements to steel to improve its properties. The most common alloying elements are carbon, manganese, silicon, chromium, and nickel. Carbon increases the strength and hardness of steel, but it also makes it more brittle.
Manganese increases the strength and toughness of steel, and it also helps to prevent corrosion. Silicon increases the strength and elasticity of steel, and it also helps to prevent oxidation. Chromium increases the hardness and wear resistance of steel, and it also helps to prevent corrosion.
Nickel increases the strength and toughness of steel, and it also helps to improve its ductility.
Heat treatment is the process of heating and cooling steel to change its properties. The most common heat treatments are annealing, normalizing, hardening, and tempering. Annealing is a process of heating steel to a high temperature and then slowly cooling it.
This process softens the steel and makes it more ductile. Normalizing is a process of heating steel to a high temperature and then cooling it in air. This process produces a steel that is stronger and harder than annealed steel, but it is also more brittle.
Hardening is a process of heating steel to a high temperature and then rapidly cooling it. This process produces a steel that is very hard and wear-resistant, but it is also very brittle. Tempering is a process of heating hardened steel to a lower temperature and then cooling it slowly.
This process reduces the hardness and brittleness of the steel, while still maintaining its strength and wear resistance.
4. Casting and Rolling

Once the steel has been alloyed and heat treated, it is cast into ingots. Ingots are large, solid blocks of steel that are typically square or rectangular in shape. The ingots are then rolled into various shapes, such as sheets, plates, bars, and rods.
Rolling is a process of passing the ingots through a series of rollers, which gradually reduce their thickness and width.
The different types of steel products available include hot-rolled steel, cold-rolled steel, and stainless steel. Hot-rolled steel is produced by rolling ingots at a high temperature. This process produces a steel that is strong and durable, but it has a rough surface finish.
Cold-rolled steel is produced by rolling hot-rolled steel at a lower temperature. This process produces a steel that has a smooth surface finish and is more precise in dimension than hot-rolled steel. Stainless steel is a type of steel that contains a high percentage of chromium.
This makes the steel resistant to corrosion and staining.
5. Applications of Steel

Steel is used in a wide variety of applications, including construction, transportation, manufacturing, and energy. In construction, steel is used to build bridges, buildings, and other structures. In transportation, steel is used to build cars, trucks, trains, and ships. In manufacturing, steel is used to make tools, machinery, and other products.
In energy, steel is used to build power plants and other energy-related structures.
Steel is a versatile material that can be used to make a wide variety of products. It is strong, durable, and relatively inexpensive. This makes it an ideal material for a wide range of applications.
The future prospects for the steel industry are bright. Steel is a vital material for modern society, and it is likely to continue to be used in a wide variety of applications for many years to come.
Question Bank
What are the key steps involved in making steel?
Steelmaking encompasses a series of crucial steps, including extracting iron from ore, refining it into pig iron, and further purifying it to produce steel. Alloying elements are then added to enhance the steel’s properties, followed by heat treatment processes to achieve desired characteristics.
How does the Bessemer process differ from the electric arc furnace process?
The Bessemer process, a pioneering method, utilizes air blown into molten iron to remove impurities. In contrast, the electric arc furnace process employs electric arcs to melt scrap metal and iron ore, offering greater control over the steel’s composition and properties.
